
Journey of Splitting H20 into H2+O2
For several years, hard-to-mine sectors such as aviation, steel, and shipping have relied on coal, oil, and natural gas. These hard-to-degrade sectors are among the world’s largest emitters of CO2 and face the challenge of complete decarbonization. Environmental changes and the depletion of fossil fuels led to the need to research and development. Of sustainable and clean alternative renewable energy sources.
Hydrogen promises to be an ideal candidate in this regard. There are several ways to generate a green hydrogen electrolyzer. Hydrogen production by water electrolysis has a significant position in the market. Green hydrogen electrolyzer are devices that produce hydrogen and oxygen by splitting water using electricity. The process is simple and produces a high level of pure gas without releasing pollutant gases into the atmosphere. The most commonly used method for hydrogen production. One electrolytic cell is a combination of various elements through which water flow with low voltage direct current is passed. Renewable energy.
There are mainly two types of electrolytic cells, namely monopolar and bipolar. In monopolar design, the electrodes are negative or positive and we electrically connected each cell in parallel, and in the bipolar design, the individual cells are electrically and geometrically connected in series, making it the preferred option for green hydrogen electrolyzer manufacturers.
Bi-polar and Mono-polar electrolysis –
Bipolar cells are more compact than monopolar systems, resulting in shorter current paths in the electrical wires and electrodes. This reduces loss due to the internal ohmic resistance of the electrolyte, thus increasing the operating efficiency of the green hydrogen electrolyzer.
In addition, mass production of bipolar electrolytic cells is technically difficult, time-consuming, and expensive. Currently, R&D needs to focus mainly on developing durable materials to extend the life and performance of electrolysis stacks. Reducing system complexity also remains a major challenge. Renewable energy.
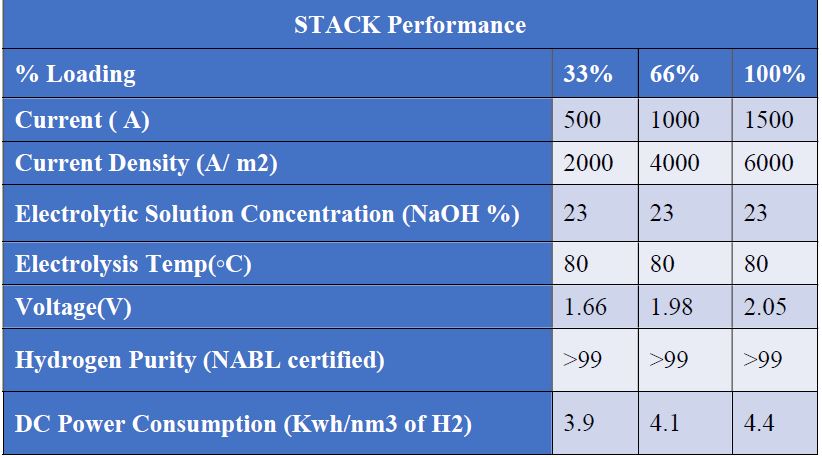
Existing electrode coating seems to be improperly leading to higher power consumption. Current cell designs are not guided, thus longer stacking becomes a challenging task. Also, non-guided stack design requires higher attention and skill. Existing designs do not address foaming issues appropriately, leading to cell drying and temperature-related issues. Metallic outer frame manufacturing of the electrolyzer is a tedious, costly, time-consuming, and non-efficient process. Hence, efforts towards simplified and low-carbon manufacturing techniques of bi-polar electrolytic cells could bring attractive benefits to this global green hydrogen electrolyzer mission.
BRISE Bi-Polar Electrolyser addresses some of these issues, offering a reliable and affordable Bi-polar electrolytic cell with an outer frame having better resistivity in electrical current and thermal conduction at the outer surface and operates at lower risk. Non-metallic molded outer edge reduces manufacturing cost and time, thereby improving production efficiency as below tabulated. Overall, less time and effort are required to manufacture and assemble the bi-polar plate with the outer frame. Renewable energy.
Life of BRISE Electrolyser
Our electrode Based material is Pure Nickel with Catalytic coating With reference to NACE Paper 13297, table 4, Mass loss due to corrosion is 0.7 microns/year at 80°C, even if we considered plane electrode made up N06625 with 100-micron thickness then the life of the electrode will be easily 20+ year at 80°C operating temperature with wt. 25% concentrated Alkaline solution at 30barg in oxidizing environments.
- Similarly, the way our SS316 Bi- polar plate coated with nickel life span will be 20+ year
- Having said that life of coating in both cases will be more than 15 + years and after that stack will keep running with pure nickel electrode.
- Gaskets may need to replace after every 5 years if leakage is identified and membranes need to replace after 10 years
- For all other system components & that was bought out such as the pump & we design valves with SS316, which is in contact with an alkaline solution with a design life of 25 years. Renewable energy.



H2 generator
Bright Future For India & Brise Chemical
We are interested in designing solar direct solution/s for Green Hydrogen electrolyser.
Shall be happy to connect
Thank you for showing interest. Please contact on projects@brisechemicals.com