Hydrogen Electrolyzer

How Does a Hydrogen Electrolyzer Work?
The water electrolyzer is a device that generates hydrogen and oxygen from the water through the application of electricity. It consists series of Porous Nikel with special surface treatment through which water flows while a low voltage direct current is applied. A water electrolyzer stack consists of several cells linked in series.
These cells are of two types namely monopolar and bipolar cells
In the monopolar design, the electrodes are either negative or positive with the parallel electrical connection of the individual cells (Fig.A), while in the bipolar design the individual cells are linked in series electrically and geometrically (Fig.B).and it is the most preferred option for water electrolyzer manufacturers. One advantage of the bipolar cell design is that they are more compact than monopolar systems which give shorter current paths in the electrical wires and electrodes. This reduces the losses due to the internal ohmic resistance of the electrolyte and therefore increases the electrolyzer efficiency. Brise Chemicals is hydrogen electrolyzer manufacturers India.
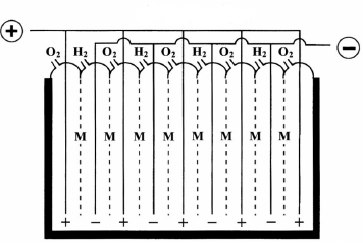
(Fig. A) Monopolar
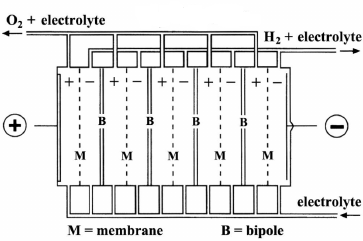
(Fig. B) Biopolar
Alkaline water electrolyzer usually uses an alkaline solution of sodium or potassium hydroxide (NaOH or KOH) that acts as the electrolyte, mostly with 20-30% of weight because of the optimal conductivity, they require using corrosion-resistant stainless steel to withstand the chemical attack. This electrolyzer operates at a temperature of 70-100°C and at a pressure of 1-30 bar. The following are the two chemical reactions that occur at the anode and cathode, finally resulting in hydrogen production.
For Cathode reaction is:
2 H2O(l) + 2e− → H2(g) + 2 OH−(aq)
For Anode reaction is:
2 OH−(aq) → 1/2 O2(g) + H2O(l) + 2 e−
And finally, the overall electrolyzer reaction is:
2 H2O(l) → 2 H2(g) + O2(g)
Brise Chemicals is OEM &Technology owner of hydrogen electrolyzer manufacturers India listed company & located at Chakan,Pune, Maharashtra,India. In Brise’s Bipolar design, where high-purity DM water is broken to H2 and O2 using DC power supplied by the rectifier. Hydrogen is evolved on the cathode side of cells and oxygen is on the anodes of cells. The design makes our Alkaline water Electrolyzer unit very compact. This specially developed cell unit produces gases at high pressure of 0-30 Bar. So, no Hydrogen compressor is needed for medium Hydrogen pressure requirements.
Produced Oxygen can be released in atmosphere or utilized for medical or industrial purpose. The hydrogen is compressed or liquefied and stored for use in industry or in hydrogen fuel cells, which can power vehicles such as trains, ships, and even aircraft.
Benefits of Hydrogen Electrolyzer
- Increased efficiency
- Environment friendly
- Dependable energy
- Safer to use
Hydrogen Electrolyzer Applications Across Industries
- Transportation Industry
- Oil And Gas Industry
- Manufacturing, Fertilizer Industry
- Steel & Chemical Industry
Product Offering
| Type | Alkaline Electrolyzer | ||||||
|---|---|---|---|---|---|---|---|
| Electrolyte | 30%NaOH | ||||||
| Stack Size(Watts) | 3.8-4.4 kWh/Nm3 | ||||||
| Hydrogen Production | 5 Nm3/h | 50 Nm3/h | 150 Nm3/h | 300 Nm3/h | 500 Nm3/h | 1000 Nm3/h | 2000 Nm3/h |
| Stack Series No. | AE-5 | AE-50 | AE-150 | AE-300 | AE-500 | AE-1000 | AE-2000 |
| KG/24 H | 11 kg/day | 107 kg/day | 324 kg/day | 647 kg/day | 1046 kg/day | 2094 kg/day | 4320 kg/day |
| Oxygen Production | 2.5 Nm3/h | 25 Nm3/h | 75 Nm3/h | 150 Nm3/h | 250 Nm3/h | 500 Nm3/h | 1000 Nm3/h |
| Cell Voltage(v) | 230-420 | ||||||
| Current Density(A/cm2) | 0.2-0.8 | ||||||
| Stack Current(A) | 100-4000 | ||||||
| Cell Active Area(cm2) | 125-20000 | ||||||
| Operating Temperature | 70-90 °C | ||||||
| Ambient Temperature | 5-35 °C | ||||||
| Operating Pressure(barg) | 1-15 barg | ||||||
| Purity(%)H2 | 99.99-99.999% | ||||||
| purity(%)O2 | 99.99-99.999% | ||||||
| Oxygen Limit | <2 ppm v | ||||||
| Moisture Content | <2 ppm v | ||||||
| *Standardised Hydrogen Generator Sizes: 5nm3/hr, 50 nm3/hr, 150 nm3/hr, 485 nm3/hr *Our design can be customised as per user requirement |
|||||||

